BFSF Continues to be an Area of Focus

Author’s note: In this article, we provide insights into critical developments in pharmaceutical pricing, recent cases, and industry trends. Should you have any questions or thoughts to share, please feel free to reach out to us, and we would be glad to help you. And finally, special thanks to Rick Moore and Caitlin Fee for their contributions for this article.
Get the PDF version of this article for offline reading or reference: Download PDF
Recent Updates, Litigation, and Settlements
As you may have heard at the recent Medicaid conference in May or the Informa Connect Fireside Chat: Hot Topics in Pricing & Contracting webinar earlier this week, there continues to be a lot of focus on pharma manufacturers and their arrangements with third party service providers. Here’s a quick summary of recent cases – we recommend reading the cases (see article pdf for sources).
1. On August 3, 2022, an Illinois federal jury hit Eli Lilly and Co. with a $61 million verdict Wednesday, after finding that the drug manufacturer deliberately shorted the Medicaid program by leaving retroactive drug price increases out of the metrics it was required to include for drug rebate calculations. This is a reminder that manufacturers need to continue to ensure that service fees and non-BFSF are evaluated and treated appropriately for U.S. government pricing calculations and that documentation of the BFSF evaluation is arguably just as important as the results (see the commentary and notes of this case). The treatment of services fees and other payments can have a significant impact on government pricing calculations including Average Manufacturer Price (AMP) which directly impacts the amount of rebates manufacturers pay.
2. Per Reuters, “Biogen Inc said it had cinched a $900 million deal to resolve a whistleblower lawsuit accusing the biotech company of paying doctors kickbacks to prescribe multiple sclerosis drugs just days before a trial was scheduled to kick off”. This is still subject to approval by the U.S. Justice Department. This is an important reminder to not only be mindful about your arrangements with physicians and speaker programs and ensuring that rates and services are fair market value, but also monitor overall activities and that it is within your company’s guidelines, reasonable, and consistent with current best practices.
3. Earlier this year, Cardinal Health, Inc., has agreed to pay $13,125,000 to resolve allegations that it violated the False Claims Act by paying “upfront discounts” to its physician practice customers, in violation of the Anti-Kickback Statute. While this case happens to be related to a distributor, it’s a reminder that the government continues to focus on this area and reducing healthcare fraud.
“Cardinal Health thought it hit upon a surefire moneymaker by paying kickbacks to doctors, which cost health benefit programs millions of dollars in potentially fraudulent claims,” said Joseph R. Bonavolonta, Special Agent in Charge of the Federal Bureau of Investigation, Boston Division. “Anyone involved in, or entertaining, similar activity should know that health care fraud is a priority for the FBI, and we will pursue anyone trying to profit from this country’s vital health care system.”
4. Johnson & Johnson recently filed a lawsuit against SaveOnSP of contract interference and deceptive trade practices. J&J is seeking damages and a court order that SaveOnSP stop its program (see sources and detail below). While the suit isn’t focused on BFSF or government pricing for that matter, it’s a reminder that manufacturers need to continue evaluating their service arrangements with patient assistance program providers and other channel intermediaries.
5. Lastly, as you may already know, late last year, three generic manufacturers agreed to pay a total of $447.2 million to resolve alleged violations of the False Claims Act arising from conspiracies to fix the price of various generic drugs.5 These CIA’s are very unique and are focused on pricing and contracting, including government pricing. As such, it’s important for manufacturers to continue to evaluate and ensure their pricing and contracting processes are following leading practices including evaluating arrangements with customers and service providers in addition to product price negotiations.
Quick Refresher on BFSF
Pursuant to 42C.F.R.§447.502, the definition of BFSF for purposes of Average Manufacturers Price (“AMP”), Best Price (“BP”), and Average Sales Price (“ASP”):
Fees paid by a manufacturer to an entity, that represent fair market value for a bona fide, itemized service actually performed on behalf of the manufacturer that the manufacturer would otherwise perform (or contract for) in the absence of the service arrangement, and that are not passed on in whole or in part to a Company or customer of an entity, whether or not the entity takes title to the drug.
BFSF Test components
- The fee is paid for a bona fide, itemized service actually performed on behalf of the manufacturer.
- The manufacturer would otherwise perform (or contract for) the service in the absence of the service arrangement.
- The fee is not passed on in whole or in part to a client or customer of the service-providing entity, whether or not the entity takes title to the drug.
- The fee represents Fair Market Value (FMV) for the service
Do BFSF still matter and why?
- BFSF analysis is relevant to:
- Government price reporting (AMP, BP, ASP, FSS)
- Reimbursement rates and remuneration within supply chain arrangements
- Gross to net reporting
- Compliance and legal exposure
- Legal risk:
- Potential civil monetary penalties
- Knowingly submitting false pricing or product data
- Misrepresentation in the reporting of ASP data
- Knowing and intentional 340B overcharges
- Potential False Claims Act and other liability
- E.g., potential Anti-Kickback Statute liability
- Enforcement:
- Settlements
- Litigation
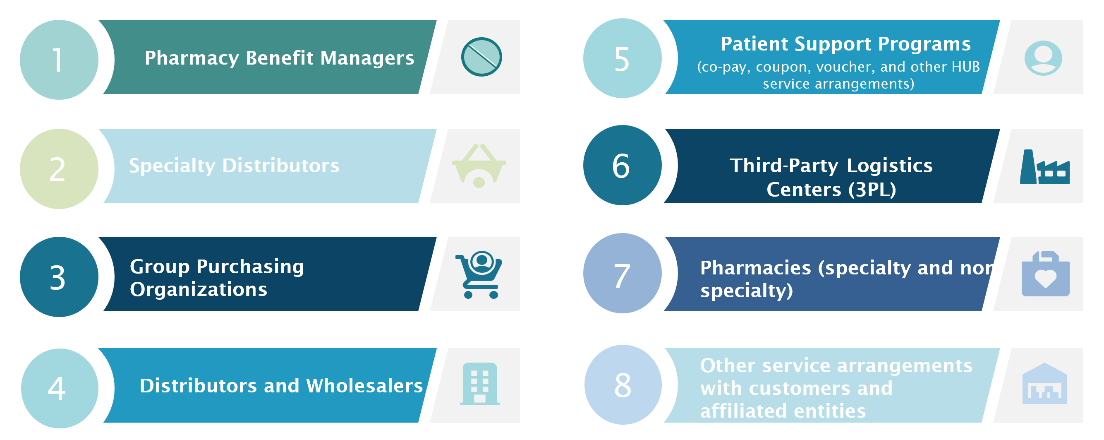
Entities that may require a BFSF Analysis
Below is an Illustrative list of service arrangement types that may require analysis. Manufacturers must determine the ultimate list of entities that require this analysis:
Hot topics, Key trends, and items to consider relating to BFSF/FMV
Hot Topics & Key Trends:
- Digitalization and technology continue to become more prominent. These range in complexity depending on assets and parties involved (especially if HCPs are using the technology and it could be viewed as a transfer of value).
- While in person meetings and events have comeback, it appears that hybrid and virtual based marketing, sponsorships, conferences, etc. are here to stay.
- Data continues to be key and the holders of data of course recognize the value to manufacturers.
- Specialty, gene therapy, and biosimilar products continue to be an area of focus and each product is unique and as such may have unique services arrangements to help ensure a smooth patient journey.
- Manufacturers are paying close attention to their service arrangements and evaluating from a BFSF, legal, risk, government pricing, and market access perspective.
- Manufacturers, counsel, and vendors, continue to be divided and challenge which activities in a service arrangement are actually services for purposes of a BFSF evaluation.
- There is increased focus on patient support programs and specialty pharmacy arrangements.
- 340B program and pricing continues to be a pain point and a key area of focus for manufacturers.
Items to Consider to Help Mitigate Risks:
- Develop processes and incorporate tools (i.e., checklist/questionnaires/FMV estimators) to help facilitate and incorporate BFSF evaluations in contracting process. This is a key item to help ensuring that service arrangements are being evaluating not only from a fair market value perspective but taking a close look at the qualitative prongs of the test (i.e., is this a service on our behalf, is their pass-through evidence or notice, services itemized in the contract) and having a framework in place for the evaluation including when to seek counsel advice. In practice, we’ve seen it work well when manufacturers incorporate BFSF review as part of the contracting process to ensure appropriate stakeholders are aligned and there are no surprises when it comes to government pricing or gross to net.
- Perform periodic BFSF/FMV training so business teams are aware of process and mindful of current rules, regulations, and that the company creates and maintains documentation of the BFSF analysis and treats the fees appropriately in government pricing.
- Monitor and track performance of service providers and overall spend and evaluate whether there’s an opportunity to mitigate risks.
- If you’re contracts allow the vendor to invoice net of service fees, discounts, and other adjustment on their invoices, ensure that you’re reviewing them and treating the fees appropriately. Note this is an area that is commonly overlooked especially as it relates to items like price appreciation credits. It’s important to note that CMS has made clear that price appreciation credits are not BFSF and should be included in calculations.
- Document your BFSF evaluation, follow your BFSF process, perform FMV, and ensure that fees are treated appropriately based on the results of the BFSF evaluation. As a reminder, there is no conservative approach for BFSF (i.e., depending on your product, what’s conservative in one government program may be aggressive in another). Note we generally recommend checking with counsel; especially for new or complex arrangements, if it’s your first evaluation, or if it’s a sensitive matter that should be performed under privilege.
- Perform periodic government pricing assessments to ensure calculations are accurate and complete, appropriate reasonable assumptions and methodology are documented, policies and procedures are current, and service fee treatment in calculations aligns to BFSF documentation and that all service arrangements are reviewed.
- If you’ve already performed FMV and BFSF evaluation, keep track and perform periodic refreshes as needed. We typically recommend every 2-3 years, unless something significantly changes (i.e., sales or service changes, new rules/guidance, etc..).



